How I assess the current COVID-19 status of Florida:
There are several public sources with data we can all use to make an assessment of the current status for the State of Florida (or any other state).
There are several aspects of an infectious disease process that are important metrics to follow to grasp the situation:
- Current level and change of infection rate
- Current mortality rate
- Hospitalization status
- Estimated current prevalence
Here is how I attempt to make an educated assessment on any particular day:
Infection Rate
I use the Florida Department of Health’s dashboard to get an overall indication of infection rate.
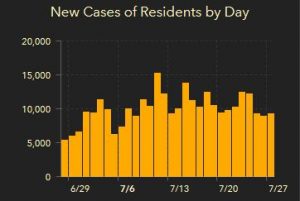
We can get a glance of the situation by looking at the new cases of residents by day. These are the numbers that make the headlines. I think these should be viewed from a big picture perspective. It is more important to watch the overall trend than the specific level each day. These numbers have some inherent inaccuracies as they will not include everyone who is infected (under count) and will also include people who are being retested to see if they can return to work (over count).
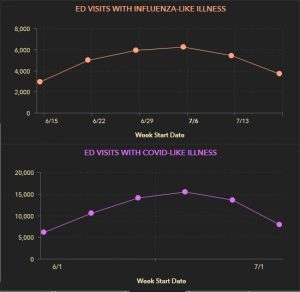
Looking at visits to Emergency Departments (ED) can give an indication of level of infection. If these visits go up, more people are possibly getting sick (maybe even increase in severity), and if they go down, the opposite is true.
Here you can see that through June these visits were going up, but since early July, they have been decreasing. This could mean fewer infected people. Not everyone needs to visit the ED for management nor need inpatient management. Fewer people requesting help, though, can indicate fewer cases among the public at large.
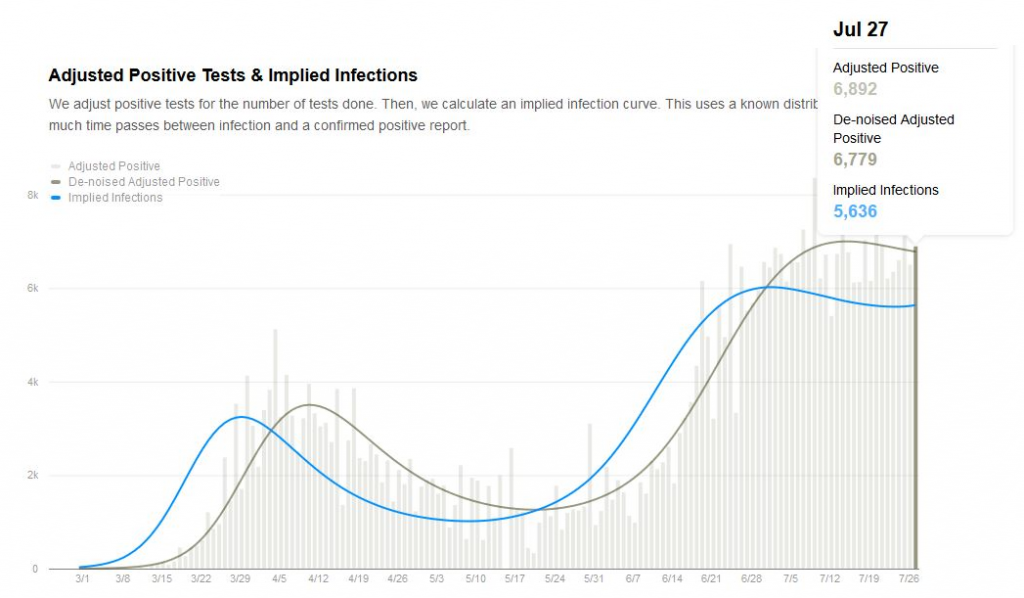
To get a full impression on infection rate, I suggest to look at the R0 estimate. Today, the R0 for Florida is 0.98, meaning COVID infections are contracting not expanding.
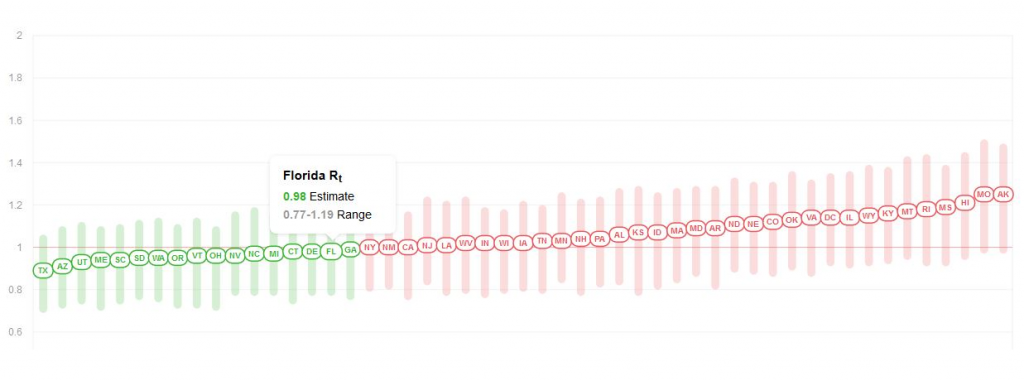
What is great about this website, their algorithm attempts to correct for testing volume giving a potentially more accurate true infection rate. For the end of July, this implied infections is more encouraging than the absolute reported positives.
Mortality Rate
COVID infections matter because people die from it. To understand the COVID situation it is also important to look at the deaths by day from COVID.
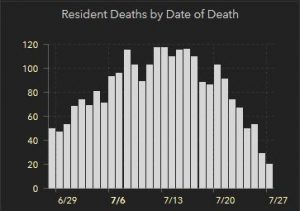
At first glance, this looks great, the death rate has dropped dramatically compared with a more flat decrease of infections. I expect there to be a real decrease here, but not as dramatic as this snapshot appears. The numbers from more recent days are under reported, as this data comes from death certificate reports. These will trickle in over the next couple of weeks making this a tough trend to monitor.
Hospitalizations
We “flattened the curve” for COVID to not overrun hospital capacity to care for the sick. As of today, we have 8,992 people hospitalized with the primary diagnosis of COVID. This number is meaningless unless we know what our available hospital bed capacity is. Today, we have 21.91% of our hospital beds available in Florida (15.95% of our ICU beds remain available).
COVID-19 Prevalence
I estimate prevalence (percent of the population who are infected) by totaling the new cases from the last 14 days (as it usually takes 10-14 days to recover) and dividing by the State population. This result is only a guide and will be inaccurate at any level more granular than the state (unless you use county or city numbers).
At this moment, I estimate the prevalence of COVID-19 in Florida at: 0.75%. This is the percent of the population in Florida with an active COVID-19 infection. This number is obviously not consistent across the state; in cities, the prevalence is much higher, while in rural areas likely much lower. In general, though, this is a very low prevalence disease.
Hopefully this helps you out as you continue precautions against COVID-19.